
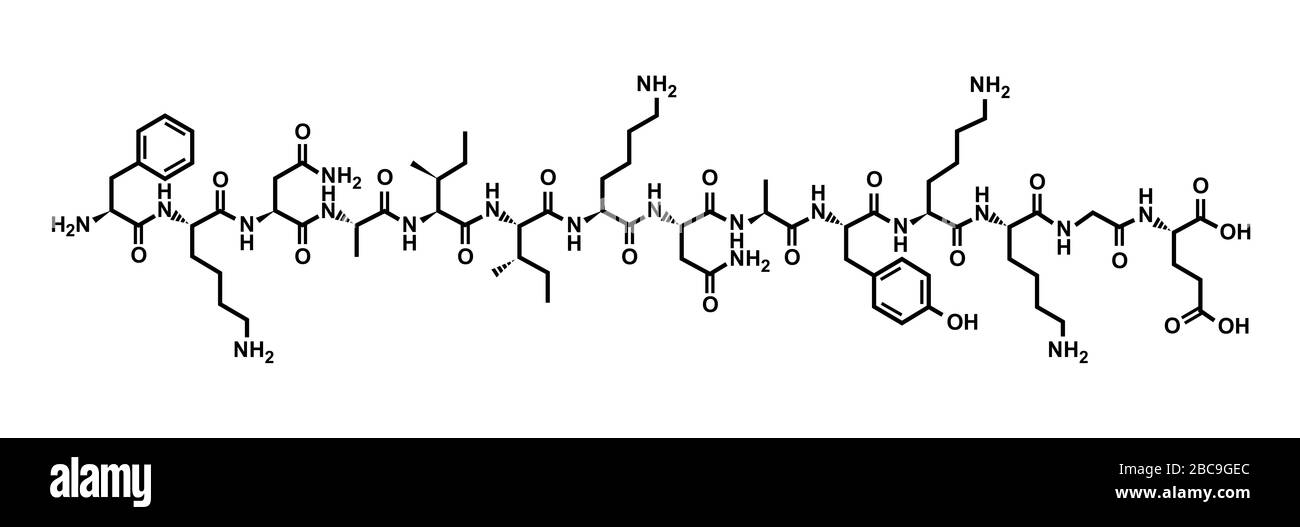
This term was essentially assigned to any peptide that demonstrated morphine-like activity. Simon, who independently discovered opioid receptors, would later term these peptides as endorphins. Snyder isolated morphine-like peptides from calf brain. Research during this time was focused on the search for a painkiller that did not have the addictive character or overdose risk of morphine. This came after the discovery of a receptor that was proposed to produce the pain-relieving analgesic effects of morphine and other opioids, which led Kosterlitz and Hughes to their discovery of the endogenous opioid ligands. They isolated " enkephalins" (from the Greek εγκέφαλος, cerebrum) from pig brain, identified as Met-enkephalin and Leu-enkephalin. Overall, our computational study reveals that the strategic positioning of ionizable residues into the cross-β core is a potential approach for designing reversible amyloid fibrils as pH-responsive smart bio-nanomaterials.Opioid peptides in the brain were first discovered in 1973 by investigators at the University of Aberdeen, John Hughes and Hans Kosterlitz. Electrostatic repulsion between deprotonated Glu8 residues along with their high solvation tendency disrupted the hydrogen bonding between the β1 strands and increased the solvent exposure of those otherwise buried residues in the cross-β core. Via iterative deprotonation of Glu8 and induced structural disruption, all Glu8 residues would be progressively deprotonated. After switching to neutral pH, the Glu8 residues of peptides at the outer layers of the ordered fibrils became deprotonated due to partial solvent exposure, causing sheet-to-coil conformational changes and subsequent exposure of adjacent Glu8 residues in the inner chains. The fibril was stable at pH 5.5 or lower with all the buried Glu8 residues protonated and neutrally charged. Here, we investigate the critical role of a buried glutamate, Glu8, in the pH-responsive disassembly of β-endorphin fibrils using all-atom molecular dynamics simulations along with structure-based p K a prediction. The environmental change between low pH in secretory granules and neutral pH in extracellular spaces is believed to drive the reversible fibrillization of β-endorphin.

β-Endorphin, an endogenous opioid peptide hormone, forms such kinds of reversible amyloid fibrils in secretory granules for efficient storage and returns to the functional state of monomers upon release into the blood. In contrast to the irreversible aggregation of most known pathological amyloids, we postulate that naturally-occurring functional amyloids are reversible under evolutionary pressure to be able to modulate the fibrillization process, reuse the composite peptides, or perform their biological functions.
Functional amyloids are abundant in living organisms from prokaryotes to eukaryotes playing diverse biological roles.


 0 kommentar(er)
0 kommentar(er)
